Introduction to Matter (Book)
| Site: | Learnbps |
| Class: | MarketPlace for Science |
| Book: | Introduction to Matter (Book) |
| Printed by: | Guest user |
| Date: | Thursday, February 26, 2026, 9:06 AM |
Description

Introduction to Matter
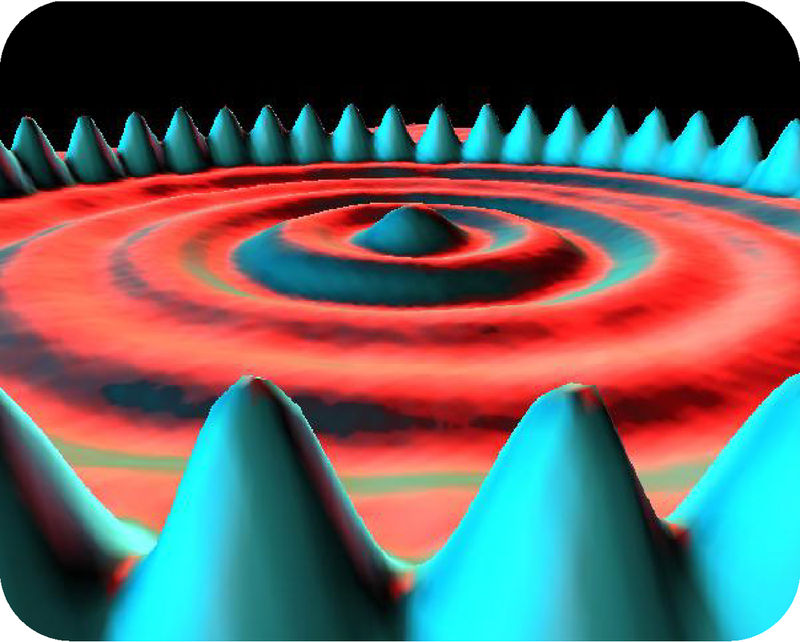 The greenish-blue peaks in this picture are individual atoms of iron on a background of copper. This amazing image was made by a scanning tunneling microscope. It’s the only kind of microscope that can make images of things as small of atoms. The invention of the scanning tunneling microscope was so significant that its inventors got a Nobel prize for it. Why is being able to see atoms so important? Atoms are the basic building blocks of all the matter in the universe. You will learn more about atoms and matter when you read this book.
The greenish-blue peaks in this picture are individual atoms of iron on a background of copper. This amazing image was made by a scanning tunneling microscope. It’s the only kind of microscope that can make images of things as small of atoms. The invention of the scanning tunneling microscope was so significant that its inventors got a Nobel prize for it. Why is being able to see atoms so important? Atoms are the basic building blocks of all the matter in the universe. You will learn more about atoms and matter when you read this book.
BPS-S
Science Standards
The following NGSS Standards will be the focus of instruction for this unit of study.
SCI-MS.PS1
Matter and Its Interactions
- SCI-MS.PS1.01 Develop models to describe the atomic composition of simple molecules and extended structures.
- SCI-MS.PS1.02 Analyze and interpret data on the properties of substances before and after the substances interact to determine if a chemical reaction has occurred.
- SCI-MS.PS1.03 Gather and make sense of information to describe that synthetic materials come from natural resources and impact society.
- SCI-MS.PS1.04 Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.
- SCI-MS.PS1.05 Develop and use a model to describe how the total number of atoms does not change in a chemical reaction and thus mass is conserved.
- SCI-MS.PS1.06 Undertake a design project to construct, test, and modify a device that either releases or absorbs thermal energy by chemical processes.
Legend
The benchmarks colored BLUE "Need to Know" indicate them as identified for targeted for all students to master, with YELLOW "Important to Know" supporting the priority benchmarks. This was a prioritization process not an elimination - the white are the overall supporting benchmarks.
Properties of Matter
Introduction
Here’s a riddle for you to ponder: What do you and a tiny speck of dust in outer space have in common? Think you know the answer? Read on to find out.
Lesson Objectives
- Define matter, mass, and volume.
- Identify physical properties of matter.
- List examples of chemical properties of matter.
- Quizlet Vocab
What is Matter?
Both you and the speck of dust consist of atoms of matter. So does the ground beneath your feet. In fact, everything you can see and touch is made of matter. The only things that aren’t matter are forms of energy, such as light and sound. Although forms of energy are not matter, the air and other substances they travel through are. So what is matter? Matter is defined as anything that has mass and volume.
- Mass
Mass is the amount of matter in a substance or object. Mass is commonly measured with a balance. A simple mechanical balance is shown in Figure below. It allows an object to be matched with other objects of known mass. SI units for mass are the kilogram, but for smaller masses grams are often used instead.
This balance shows one way of measuring mass. When both sides of the balance are at the same level, it means that objects in the two pans have the same mass.
- Mass versus Weight
The more matter an object contains, generally the more it weighs. However, weight is not the same thing as mass. Weight is a measure of the force of gravity pulling on an object. It is measured with a scale, like the kitchen scale in Figure below. The scale detects how forcefully objects in the pan are being pulled downward by the force of gravity. The SI unit for weight is the newton (N). The common English unit is the pound (lb). With Earth’s gravity, a mass of 1 kg has a weight of 9.8 N (2.2 lb).
This kitchen scale measures weight. How does weight differ from mass?
Physical Properties of Matter
Matter has many properties. Some are physical properties. Physical properties of matter are properties that can be measured or observed without matter changing to a different substance. For example, whether a given substance normally exists as a solid, liquid, or gas is a physical property. Consider water. It is a liquid at room temperature, but if it freezes and changes to ice, it is still water. Generally, physical properties are things you can see, hear, smell, or feel with your senses.
Examples of Physical Properties
Physical properties include the state of matter and its color and odor. For example, oxygen is a colorless, odorless gas. Chlorine is a greenish gas with a strong, sharp odor. Other physical properties include hardness, freezing and boiling points, the ability to dissolve in other substances, and the ability to conduct heat or electricity. These properties are demonstrated in Figure below. Can you think of other physical properties?
These are just a few of the physical properties of matter.
- Density
Density is an important physical property of matter. It reflects how closely packed the particles of matter are. Density is calculated from the amount of mass in a given volume of matter, using the formula:
Chemical Properties of Matter
Some properties of matter can be measured or observed only when matter undergoes a change to become an entirely different substance. These properties are called chemical properties. They include flammability and reactivity.
- Flammability
Flammability is the ability of matter to burn. Wood is flammable; iron is not. When wood burns, it changes to ashes, carbon dioxide, water vapor, and other gases. After burning, it is no longer wood.
- Reactivity
Reactivity is the ability of matter to combine chemically with other substances. For example, iron is highly reactive with oxygen. When it combines with oxygen, it forms the reddish powder called rust (see Figure below). Rust is not iron but an entirely different substance that consists of both iron and oxygen.
The iron in this steel chain has started to rust.
Lesson Summary
- Matter is anything that has mass and volume. Mass is the amount of matter in a substance. Volume is the amount of space matter takes up.
- Matter has both physical and chemical properties. Physical properties can be measured or observed without matter changing to a different substance.
- Chemical properties of matter can be measured or observed only when matter undergoes a change to become an entirely different substance.
Types of Matter
Introduction
The properties of matter, both physical and chemical, depend on the substances that matter is made of. Matter can exist either as a pure substance or as a combination of different substances.
Lesson Objectives
- Describe elements and atoms.
- Describe compounds, molecules, and crystals.
- Define mixture, and identify types of mixtures.
- Quizlet Vocab
Elements
An element is a pure substance. It cannot be separated into any other substances. There are more than 90 different elements that occur in nature. Some are much more common than others. Hydrogen is the most common element in the universe. Oxygen is the most common element in Earth’s crust. Figure below shows other examples of elements.
Still others are described in the video below.
Each of the elements described here has different uses because of its properties.
Properties of Elements
Each element has a unique set of properties that make it different from all other elements. As a result, elements can be identified by their properties. For example, the elements iron and nickel are both metals that are good conductors of heat and electricity. However, iron is attracted by a magnet, whereas nickel is not. How could you use this property to separate iron objects from nickel objects?
History of Elements
The idea of elements is not new. It dates back about 2500 years to ancient Greece. The ancient Greek philosopher Aristotle thought that all matter consists of just four elements. He identified the elements as earth, air, water, and fire. He thought that different kinds of matter contain only these four elements but in different combinations.
Aristotle’s ideas about elements were accepted for the next 2000 years. Then, scientists started discovering the many unique substances we call elements today. You can read when and how each of the elements was discovered at the link below. Scientists soon realized that there are far more than just four elements. Eventually, they discovered a total of 92 naturally occurring elements.
http://www.rsc.org/periodic-table/history
Compounds
There are millions of different substances in the world. That’s because elements can combine in many different ways to form new substances. In fact, most elements are found in compounds. A compound is a unique substance that forms when two or more elements combine chemically. An example is water, which forms when hydrogen and oxygen combine chemically. A compound always has the same components in the same proportions. It also has the same composition throughout. You can learn more about compounds and how they form by watching this video:
Elements and Atoms
The smallest particle of an element that still has the element’s properties is an atom. All the atoms of an element are alike, and they are different from the atoms of all other elements. For example, atoms of gold are the same whether they are found in a gold nugget or a gold ring (see Figure below). All gold atoms have the same structure and properties.
Gold is gold no matter where it is found because all gold atoms are alike.
PhET Activity
Build an Atom
|
Click on image to build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas!
|
Properties of Compounds
A compound has different properties than the substances it contains. For example, hydrogen and oxygen are gases at room temperature. But when they combine chemically, they form liquid water. Another example is table salt, or sodium chloride. It contains sodium and chlorine. Sodium is a silvery solid that reacts explosively with water, and chlorine is a poisonous gas (see Figure below). But together, sodium and chlorine form a harmless, unreactive compound that you can safely sprinkle on food.
Table salt is much different than its components. What are some of its properties?
Molecules and Crystals
The smallest particle of a compound that still has the compound’s properties is a molecule. A molecule consists of two or more atoms that are joined together. For example, a molecule of water consists of two hydrogen atoms joined to one oxygen atom (see Figure below). You can learn more about molecules at this link: http://www.nyhallsci.org/marvelousmolecules/marveloussub.html.
Water is a compound that forms molecules. Each water molecule consists of two atoms of hydrogen (white) and one atom of oxygen (red).
Some compounds form crystals instead of molecules. A crystal is a rigid, lattice-like framework of many atoms bonded together. Table salt is an example of a compound that forms crystals (see Figure below). Its crystals are made up of many sodium and chloride ions. Ions are electrically charged forms of atoms.
You can actually watch crystals forming in this video:
A crystal of table salt has a regular, repeating pattern of ions.
Mixtures
Not all combined substances are compounds. Some are mixtures. A mixture is a combination of two or more substances in any proportion. The substances in a mixture may be elements or compounds. The substances don’t combine chemically to form a new substance, as they do in a compound. Instead, they keep their original properties and just intermix. Examples of mixtures include salt and water in the ocean and gases in the atmosphere. Other examples are pictured in Figure below.
All these substances are mixtures. How do they differ from compounds?
Homogeneous and Heterogeneous Mixtures
Some mixtures are homogeneous. This means they have the same composition throughout. An example is salt water in the ocean. Ocean water everywhere is about 3.5 percent salt.
Some mixtures are heterogeneous. This means they vary in their composition. An example is trail mix. No two samples of trail mix, even from the same package, are likely to be exactly the same. One sample might have more raisins, another might have more nuts.
Particle Size in Mixtures
Mixtures have different properties depending on the size of their particles. Three types of mixtures based on particle size are described below. Figure below shows examples of each type. You can watch videos about the three types of mixtures at these links:
- A solution is a homogeneous mixture with tiny particles. An example is salt water. The particles of a solution are too small to reflect light. As a result, you cannot see them. That’s why salt water looks the same as pure water. The particles of solutions are also too small to settle or be filtered out of the mixture.
- A suspension is a heterogeneous mixture with large particles. An example is muddy water. The particles of a suspension are big enough to reflect light, so you can see them. They are also big enough to settle or be filtered out. Anything that you have to shake before using, such as salad dressing, is usually a suspension.
- A colloid is a homogeneous mixture with medium-sized particles. Examples include homogenized milk and gelatin. The particles of a colloid are large enough to reflect light, so you can see them. But they are too small to settle or filter out of the mixture.
These three mixtures differ in the size of their particles. Which mixture has the largest particles? Which has the smallest particles?
Separating Mixtures
The components of a mixture keep their own identity when they combine. Therefore, they usually can be easily separated again. Their different physical properties are used to separate them. For example, oil is less dense than water, so a mixture of oil and water can be separated by letting it stand until the oil floats to the top. Other ways of separating mixtures are shown in Figure below and in the videos below.
- (2:30)
- (2:41)
Separating the components of a mixture depends on their physical properties. Which physical property is used in each example shown here?
Lesson Summary
- Elements are pure substances with unique properties. There are more than 100 different elements (92 of which occur naturally). The smallest particles of elements are atoms.
- Compounds are unique substances that form when two or more elements combine chemically. The smallest particles of compounds are molecules. Some compounds form crystals instead.
Changes in Matter
Introduction
You hit a baseball out of the park and head for first base. You’re excited. The score is tied, and now your team has a chance of getting a winning home run. Then you hear a crash. Oh no! The baseball hit a window in a neighboring house. The glass has a big hole in it, surrounded by a web of cracks (see Figure below). The glass has changed. It’s been broken into jagged pieces. But the glass is still glass. Breaking the window is an example of a physical change in matter.
When glass breaks, its physical properties change. Instead of one solid sheet of glass, it now has holes and cracks.
Lesson Objectives
- Define and give examples of physical changes in matter.
- Define and give examples of chemical changes in matter.
- State the law of conservation of mass.
- Quizlet Vocab
Physical Changes in Matter
A physical change in matter is a change in one or more of matter’s physical properties. Glass breaking is just one example of a physical change. Some other examples are shown in Figure below and in the video below. In each example, matter may look different after the change occurs, but it’s still the same substance with the same chemical properties. For example, smaller pieces of wood have the ability to burn just as larger logs do.
In each of these changes, only the physical properties of matter change. The chemical properties remain the same.
Because the type of matter remains the same with physical changes, the changes are often easy to undo. For example, braided hair can be unbraided again. Melted chocolate can be put in a fridge to re-harden. Dissolving salt in water is also a physical change. How do you think you could undo it?
Chemical Changes in Matter
Did you ever make a "volcano," like the one in Figure below, using baking soda and vinegar? What happens when the two substances combine? They produce an eruption of foamy bubbles. This happens because of a chemical change. A chemical change occurs when matter changes chemically into an entirely different substance with different chemical properties. When vinegar and baking soda combine, they form carbon dioxide, a gas that causes the bubbles. It’s the same gas that gives soft drinks their fizz.
This girl is pouring vinegar on baking soda. This causes a bubbling "volcano."
Not all chemical changes are as dramatic as this "volcano." Some are slower and less obvious. Figure below and the video below show other examples of chemical changes.
These chemical changes all result in the formation of new substances with different chemical properties. Do you think any of these changes could be undone?
Signs of Chemical Change
How can you tell whether a chemical change has occurred? Often, there are clues. Several are demonstrated in the video below.
To decide whether a chemical change has occurred, look for these signs:
- Gas bubbles are released. (Example: Baking soda and vinegar mix and produce bubbles.)
- Something changes color. (Example: Leaves turn from green to other colors.)
- An odor is produced. (Example: Logs burn and smell smoky.)
- A solid comes out of a solution. (Example: Eggs cook and a white solid comes out of the clear liquid part of the egg.)
Reversing Chemical Changes
Because chemical changes produce new substances, they often cannot be undone. For example, you can’t change a fried egg back to a raw egg. Some chemical changes can be reversed, but only by other chemical changes. For example, to undo the tarnish on copper pennies, you can place them in vinegar. The acid in the vinegar reacts with the tarnish. This is a chemical change that makes the pennies bright and shiny again. You can try this yourself at home to see how well it works.
Conservation of Mass
If you build a campfire, like the one in Figure below, you start with a large stack of sticks and logs. As the fire burns, the stack slowly shrinks. By the end of the evening, all that’s left is a small pile of ashes. What happened to the matter that you started with? Was it destroyed by the flames? It may seem that way, but in fact, the same amount of matter still exists. The wood changed not only to ashes but also to carbon dioxide, water vapor, and other gases. The gases floated off into the air, leaving behind just the ashes.
Burning is a chemical process. Is mass destroyed when wood burns?
Assume you had measured the mass of the wood before you burned it. Assume you had also trapped the gases released by the burning wood and measured their mass and the mass of the ashes. What would you find? The ashes and gases combined have the same mass as the wood you started with.
This example illustrates the law of conservation of mass. The law states that matter cannot be created or destroyed. Even when matter goes through physical or chemical changes, the total mass of matter always remains the same. (In the chapter Nuclear Chemistry, you will learn about nuclear reactions, in which mass is converted into energy. But other than that, the law of conservation of mass holds.) For a fun challenge, try to apply the law of conservation of mass to a scene from a Harry Potter film at this link:
Lesson Summary
- Physical changes are changes in the physical properties of matter but not in the makeup of matter. An example of a physical change is glass breaking.
- Chemical changes are changes in the makeup and chemical properties of matter. An example of a chemical change is wood burning.
- Matter cannot be created or destroyed even when it changes. This is the law of conservation of mass.