Earth's Minerals (Book)
| Site: | Learnbps |
| Class: | MarketPlace for Science |
| Book: | Earth's Minerals (Book) |
| Printed by: | Guest user |
| Date: | Sunday, February 15, 2026, 7:46 AM |
Description

Introduction
Scientists have discovered more than 4,000 minerals in Earth’s crust. Some minerals are found in very large amounts. Most minerals are found in small amounts. Some are very rare. Some are common. Many minerals are useful. Modern society depends on minerals and rocks that are mined. Mining is difficult work, but is necessary for us to have the goods we use.
Lessons
- Minerals
- Identification of Minerals
MINERALS
You use objects that are made from minerals every day, even if you do not realize it. You are actually eating a mineral when you eat food that contains salt. You are drinking from a mineral when you drink from a glass. You might wear silver jewelry. The shiny metal silver, the white grains of salt, and the clear glass may not seem to have much in common, but they are all made from minerals (Figure). Silver is a mineral. Table salt is the mineral halite. Glass is produced from the mineral quartz
Just looking at that list you see that minerals are very different from each other. If minerals are so different, what do all minerals have in common?
Silver is used to make sterling silver jewelry. Table salt is the mineral halite. Glass is produced from the mineral quartz.
Standards
- SCI-MS.ESS2.01 Develop a model to describe the cycling of Earth’s materials and the flow of energy that drives this process.
Vocabulary
Lesson Objectives
- Describe the properties that all minerals share.
- Describe some different crystal structures of minerals.
- Identify the groups in which minerals are classified.
CK-12 Earth Science For Middle Schools
Mineral Resources 
+ What is Matter?
To understand minerals, we must first understand matter. Matter is the substance that physical objects are made of.
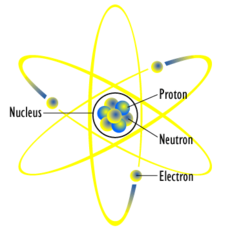
Atoms and Elements
An atom is the smallest unit of a chemical element. That is, an atom has all the properties of that element. All atoms of the same element have the same number of protons.
Molecules and Compounds
A molecule is the smallest unit of a chemical compound. A compound is a substance made of two or more elements. The elements in a chemical compound are always present in a certain ratio.
Water is probably one of the simplest compounds that you know. A water molecule is made of two hydrogen atoms and one oxygen atom (Figure). All water molecules have the same ratio: two hydrogen atoms to one oxygen atom.
A water molecule has two hydrogen atoms (shown in gray) bonded to one oxygen molecule (shown in red).
A water molecule has two hydrogen atoms (shown in gray) bonded to one oxygen molecule (shown in red).
+ What are Minerals?
A mineral is a solid material that forms by a natural process. A mineral can be made of an element or a compound. It has a specific chemical composition that is different from other minerals. One mineral's physical properties differ from others'. These properties include crystal structure, hardness, density and color. Each is made of different elements. Each has different physical properties. For example, silver is a soft, shiny metal. Salt is a white, cube-shaped crystal. Diamond is an extremely hard, translucent crystal.
Natural Processes
Minerals are made by natural processes. The processes that make minerals happen in or on the Earth. For example, when hot lava cools, mineral crystals form. Minerals also precipitate from water. Some minerals grow when rocks are exposed to high pressures and temperatures.
Could something like a mineral be made by a process that was not natural? People make gemstones in a laboratory. Synthetic diamond is a common one. But that stone is not a mineral. It was not formed by a natural process.
Inorganic Substances
A mineral is an inorganic substance. It was not made by living organisms. Organic substances contain carbon. Some organic substances are proteins, carbohydrates, and oils. Everything else is inorganic. In a few cases, living organisms make inorganic materials. The calcium carbonate shells made by marine animals are inorganic.
Definite Composition
All minerals have a definite chemical makeup. A few minerals are made of only one kind of element. Silver is a mineral made only of silver atoms. Diamond and graphite are both made only of the element carbon.
Minerals that are not pure elements are made of chemical compounds. For example, the mineral quartz is made of the compound silicon dioxide, or SiO2. This compound has one atom of the element silicon for every two atoms of the element oxygen.
Each mineral has its own unique chemical formula. For example, the mineral hematite has two iron atoms for every three oxygen atoms. The mineral magnetite has three iron atoms for every four oxygen atoms. Many minerals have very complex chemical formulas that include several elements. However, even in more complicated compounds, the elements occur in definite ratios.
Solid Crystals
Minerals must be solid. For example, ice and water have the same chemical composition. Ice is a solid, so it is a mineral. Water is a liquid, so it is not a mineral.
Some solids are not crystals. Glass, or the rock obsidian, are solid but not crystals. In a crystal, the atoms are arranged in a pattern. This pattern is regular and it repeats. Figure shows how the atoms are arranged in halite (table salt). Halite contains atoms of sodium and chlorine in a pattern. Notice that the pattern goes in all three dimensions.
Sodium ions (purple balls) bond with chloride ions (green balls) to form halite crystals.
The pattern of atoms in all halite is the same. Think about all of the grains of salt that are in a salt shaker. The atoms are arranged in the same way in every piece of salt.
Sometimes two different minerals have the same chemical composition. But they are different minerals because they have different crystal structures. Diamonds are beautiful gemstones because they are very pretty and very hard. Graphite is the “lead” in pencils. It's not hard at all! Amazingly, both are made just of carbon. Compare the diamond with the pencil lead in Figure below. Why are they so different? The carbon atoms in graphite bond to form layers. The bonds between each layer are weak. The carbon sheets can just slip past each other. The carbon atoms in diamonds bond together in all three directions. This strong network makes diamonds very hard.
Diamonds (A) and graphite (B) are both made of only carbon, but they're not much alike.
Physical Properties
The patterns of atoms that make a mineral affect its physical properties. A mineral’s crystal shape is determined by the way the atoms are arranged. For example, you can see how atoms are arranged in halite in Figure above. You can see how salt crystals look under a microscope in Figure below. Salt crystals are all cubes whether they're small or large.
Under a microscope, salt crystals are cubes.
Other physical properties help scientists identify different minerals. They include:
- Color: the color of the mineral.
- Streak: the color of the mineral’s powder.
- Luster: the way light reflects off the mineral’s surface.
- Specific gravity: how heavy the mineral is relative to the same volume of water.
- Cleavage: the mineral’s tendency to break along flat surfaces.
- Fracture: the pattern in which a mineral breaks.
- Hardness: what minerals it can scratch and what minerals can scratch it.
+ Groups of Minerals
Imagine you are in charge of organizing more than 100 minerals for a museum exhibit. People can learn a lot more if they see the minerals together in groups. How would you group the minerals together in your exhibit?
Mineralogists are scientists who study minerals. They divide minerals into groups based on chemical composition. Even though there are over 4,000 minerals, most minerals fit into one of eight mineral groups. Minerals with similar crystal structures are grouped together.
Silicate Minerals
One silicon atom bonds to four oxygen atoms to form a pyramid
About 1,000 silicate minerals are known. This makes silicates the largest mineral group. Silicate minerals make up over 90 percent of Earth's crust!
Silicates contain silicon atoms and oxygen atoms. One silicon atom is bonded to four oxygen atoms. These atoms form a pyramid (Figure). The silicate pyramid is the building block of silicate minerals. Most silicates contain other elements. These elements include calcium, iron, and magnesium.
Silicate minerals are divided into six smaller groups. In each group, the silicate pyramids join together differently. The pyramids can stand alone. They can form into connected circles called rings. Some pyramids link into single and double chains. Others form large, flat sheets. Some join in three dimensions.
Feldspar and quartz are the two most common silicates. In beryl, the silicate pyramids join together as rings. Biotite is mica. It can be broken apart into thin, flexible sheets. Compare the beryl and the biotite shown in Figure.
Beryl (a) and biotite (b) are both silicate minerals.
Native Elements
Native elements contain only atoms of one type of element. They are not combined with other elements. There are very few examples of these types of minerals. Some native elements are rare and valuable. Gold, silver, sulfur, and diamond are examples.
Carbonates
What do you guess carbonate minerals contain? If you guessed carbon, you would be right! All carbonates contain one carbon atom bonded to three oxygen atoms. Carbonates may include other elements. A few are calcium, iron, and copper.
Carbonate minerals are often found where seas once covered the land. Some carbonate minerals are very common. Calcite contains calcium, carbon, and oxygen. Have you ever been in a limestone cave or seen a marble tile? Calcite is in both limestone and marble. Azurite and malachite are also carbonate minerals, but they contain copper instead of calcium. They are not as common as calcite. They are used in jewelry. You can see in Figure that they are very colorful.
The deep blue mineral is azurite and the green is malachite. Both of these carbonate minerals are used for jewelry.
Halides
Halide minerals are salts. They form when salt water evaporates. This mineral class includes more than just table salt. Halide minerals may contain the elements fluorine, chlorine, bromine, or iodine. Some will combine with metal elements. Common table salt is a halide mineral that contains the elements chlorine and sodium. Fluorite is a type of halide that contains fluorine and calcium. Fluorite can be found in many colors. If you shine an ultraviolet light on fluorite, it will glow!
Oxides
Earth’s crust contains a lot of oxygen. The oxygen combines with many other elements to create oxide minerals. Oxides contain one or two metal elements combined with oxygen. Oxides are different from silicates because they do not contain silicon. Many important metals are found as oxides. For example, hematite and magnetite are both oxides that contain iron. Hematite (Fe2O3) has a ratio of two iron atoms to three oxygen atoms. Magnetite (Fe3O4) has a ratio of three iron atoms to four oxygen atoms. Notice that the word “magnetite” contains the word “magnet”. Magnetite is a magnetic mineral.
Sulfates
Sulfate minerals contain sulfur atoms bonded to oxygen atoms. Like halides, they can form in places where salt water evaporates. Many minerals belong in the sulfate group, but there are only a few common sulfate minerals. Gypsum is a common sulfate mineral that contains calcium, sulfate, and water. Gypsum is found in various forms. For example, it can be pink and look like it has flower petals. However, it can also grow into very large white crystals. Gypsum crystals that are 11 meters long have been found. That is about as long as a school bus! Gypsum also forms at the Mammoth Hot Springs in Yellowstone National Park, shown in Figure.
Gypsum is the white mineral that is common around hot springs. This is Mammoth Hot Springs in Yellowstone National Park.
Sulfides
Sulfides contain metal elements combined with sulfur. Sulfides are different from sulfates. They do not contain oxygen. Pyrite is a common sulfide mineral. It contains iron combined with sulfur. Pyrite is also known as “fool’s gold.” Gold miners have mistaken pyrite for gold because pyrite has a greenish gold color.
+ Lesson Summary
- A mineral is a naturally occurring inorganic solid. It has a definite composition and crystal structure.
- The atoms in minerals are arranged in regular, repeating patterns.
- These patterns are responsible for a mineral's physical properties.
- Minerals are divided into groups.
- The groups are based on their chemical composition. Silicates are the most common minerals.
IDENTIFICATION OF MINERALS
How could you describe your shirt when you are talking to your best friend on the phone? You might describe the color, the way the fabric feels, and the length of the sleeves. These are all physical properties of your shirt. If you did a good job describing your shirt, your friend would recognize the shirt when you wear it. Minerals also have physical properties that are used to identify them.
Standards
- SCI-MS.ESS2.01 Develop a model to describe the cycling of Earth’s materials and the flow of energy that drives this process.
Vocabulary
Lesson Objectives
- Explain how minerals are identified.
- Describe how color, luster, and streak are used to identify minerals.
- Summarize specific gravity.
- Explain how the hardness of a mineral is measured.
- Describe the properties of cleavage and fracture.
- Identify additional properties that can be used to identify some minerals.
CK-12 Earth Science For Middle Schools
Identification of Mineral Resources 
+ How are Minerals Identified?
Imagine you were given a mineral sample similar to the one shown in Figure. How would you try to identify your mineral? You can observe some properties by looking at the mineral. For example, you can see that its color is beige. The mineral has a rose-like structure. But you can't see all mineral properties. You need to do simple tests to determine some properties. One common one is how hard the mineral is. You can use a mineral’s properties to identify it. The mineral’s physical properties are determined by its chemical composition and crystal structure.
You can use properties of a mineral to identify it. The color and rose-like structure of this mineral mean that it is gypsum.
Color, Streak, and Luster
Diamonds have many valuable properties. Diamonds are extremely hard and are used for industrial purposes. The most valuable diamonds are large, well-shaped and sparkly. Turquoise is another mineral that is used in jewelry because of its striking greenish-blue color. Many minerals have interesting appearances. Specific terms are used to describe the appearance of minerals.
- Color
Color is probably the easiest property to observe. Unfortunately, you can rarely identify a mineral only by its color. Sometimes, different minerals are the same color. For example, you might find a mineral that is a gold color, and so think it is gold. But it might actually be pyrite, or “fool's gold,” which is made of iron and sulfide. It contains no gold atoms.
A certain mineral may form in different colors. Figure shows four samples of quartz, including one that is colorless and one that is purple. The purple color comes from a tiny amount of iron. The iron in quartz is a chemical impurity. Iron is not normally found in quartz. Many minerals are colored by chemical impurities. Other factors can also affect a mineral’s color. Weathering changes the surface of a mineral. Because color alone is unreliable, geologists rarely identify a mineral just on its color. To identify most minerals, they use several properties.
Quartz comes in many different colors including: (A) transparent quartz, (B) blue agate, (C) rose quartz, and (D) purple amethyst.
- Streak
Streak is the color of the powder of a mineral. To do a streak test, you scrape the mineral across an unglazed porcelain plate. The plate is harder than many minerals, causing the minerals to leave a streak of powder on the plate. The color of the streak often differs from the color of the larger mineral sample, as Figure shows.
Streak is more reliable than color to identify minerals. The color of a mineral may vary. Streak does not vary. Also, different minerals may be the same color, but they may have a different color streak. For example, samples of hematite and galena can both be dark gray. They can be told apart because hematite has a red streak and galena has a gray streak.
Rub a mineral across an unglazed porcelain plate to see its streak. The hematite shown here has a red streak.
- Luster
Luster describes the way light reflects off of the surface of the mineral. You might describe diamonds as sparkly or pyrite as shiny. But mineralogists have special terms to describe luster. They first divide minerals into metallic and non-metallic luster. Minerals that are opaque and shiny, like pyrite, are said to have a “metallic” luster. Minerals with a “non-metallic” luster do not look like metals. There are many types of non-metallic luster. Six are described in Table.
| Non-Metallic Luster | Appearance |
|---|---|
| Adamantine | Sparkly |
| Earthy | Dull, clay-like |
| Pearly | Pearl-like |
| Resinous | Like resins, such as tree sap |
| Silky | Soft-looking with long fibers |
| Vitreous | Glassy |
Can you match the minerals in Figure with the correct luster from Table without looking at the caption?
(A) Diamonds have an adamantine luster. These minerals are transparent and highly reflective. (B) Kaolinite is a clay with a dull or earthy luster. (C) Opal’s luster is greasy. (D) Chalcopyrite, like its cousin pyrite, has metallic luster. (E) Stilbite (orange) has a resinous luster. (F) The white ulexite has silky luster. (G) Sphalerite has a submetallic luster. (H) This Mayan artifact is carved from jade. Jade is a mineral with a waxy luster.
Density and Hardness
You are going to visit a friend. You fill one backpack with books so you can study later. You stuff your pillow into another backpack that is the same size. Which backpack will be easier to carry? Even though the backpacks are the same size, the bag that contains your books is going to be much heavier. It has a greater density than the backpack with your pillow.
- Density
Density describes how much matter is in a certain amount of space. Substances that have more matter packed into a given space have higher densities. The water in a drinking glass has the same density as the water in a bathtub or swimming pool. All substances have characteristic densities, which does not depend on how much of a substance you have.
Mass is a measure of the amount of matter in an object. The amount of space an object takes up is described by its volume. The density of an object depends on its mass and its volume. Density can be calculated using the following equation:
Density=Mass/Volume
Samples that are the same size, but have different densities, will have different masses. Gold has a density of about 19 g/cm3. Pyrite has a density of only about 5 g/cm3. Quartz is even less dense than pyrite, and has a density of 2.7 g/cm3. If you picked up a piece of pyrite and a piece of quartz that were the same size, the pyrite would seem almost twice as heavy as the quartz.
- Hardness
Hardness is a mineral’s ability to resist being scratched. Minerals that are not easily scratched are hard. You test the hardness of a mineral by scratching its surface with a mineral of a known hardness. Mineralogists use the Mohs Hardness Scale, shown in Table below, as a reference for mineral hardness. The scale lists common minerals in order of their relative hardness. You can use the minerals in the scale to test the hardness of an unknown mineral.
Mohs Hardness Scale
As you can see, diamond is a 10 on the Mohs Hardness Scale. Diamond is the hardest mineral; no other mineral can scratch a diamond. Quartz is a 7. It can be scratched by topaz, corundum, and diamond. Quartz will scratch minerals that have a lower number on the scale. Fluorite is one. Suppose you had a piece of pure gold. You find that calcite scratches the gold. Gypsum does not. Gypsum has a hardness of 2 and calcite is a 3. That means the hardness of gold is between gypsum and calcite. So the hardness of gold is about 2.5 on the scale. A hardness of 2.5 means that gold is a relatively soft mineral. It is only about as hard as your fingernail.
| Hardness | Mineral |
|---|---|
| 1 | Talc |
| 2 | Gypsum |
| 3 | Calcite |
| 4 | Fluorite |
| 5 | Apatite |
| 6 | Orthoclase feldspar |
| 7 | Quartz |
| 8 | Topaz |
| 9 | Corundum |
| 10 | Diamond |
Cleavage and Fracture
Different types of minerals break apart in their own way. Remember that all minerals are crystals. This means that the atoms in a mineral are arranged in a repeating pattern. This pattern determines how a mineral will break. When you break a mineral, you break chemical bonds. Because of the way the atoms are arranged, some bonds are weaker than other bonds. A mineral is more likely to break where the bonds between the atoms are weaker.
- Cleavage
Cleavage is the tendency of a mineral to break along certain planes. When a mineral breaks along a plane it makes a smooth surface. Minerals with different crystal structures will break or cleave in different ways, as in Figure. Halite tends to form cubes with smooth surfaces. Mica tends to form sheets. Fluorite can form octahedrons.
Minerals with different crystal structures have a tendency to break along certain planes.
Minerals can form various shapes. Polygons are shown in Figure. The shapes form as the minerals are broken along their cleavage planes. Cleavage planes determine how the crystals can be cut to make smooth surfaces. People who cut gemstones follow cleavage planes. Diamonds and emeralds can be cut to make beautiful gemstones.
Cubes have six sides that are all the same size square. All of the angles in a cube are equal to 90°. Rhombohedra also have six sides, but the sides are diamond-shaped. Octahedra have eight sides that are all shaped like triangles.
- Fracture
Fracture describes how a mineral breaks without any pattern. A fracture is uneven. The surface is not smooth and flat. You can learn about a mineral from the way it fractures. If a mineral splinters like wood, it may be fibrous. Some minerals, such as quartz, fracture to form smooth, curved surfaces. A mineral that broke forming a smooth, curved surface is shown in Figure.
This mineral formed a smooth, curved surface when it fractured.
Other Identifying Characteristics
Minerals have other properties that can be used for identification. For example, a mineral’s shape may indicate its crystal structure. Sometimes crystals are too small to see. Then a mineralogist may use a special instrument to find the crystal structure.
Some minerals have unique properties. These can be used to the minerals. Some of these properties are listed in Table. An example of a mineral that has each property is also listed.
| Property | Description | Example of Mineral |
| Fluorescence | Mineral glows under ultraviolet light | Fluorite |
| Magnetism | Mineral is attracted to a magnet | Magnetite |
| Radioactivity | Mineral gives off radiation that can be measured with Geiger counter | Uraninite |
| Reactivity | Bubbles form when mineral is exposed to a weak acid | Calcite |
| Smell | Some minerals have a distinctive smell | Sulfur (smells like rotten eggs) |
+ Lesson Summary
- You can identify a mineral by its appearance and other properties.
- The color and luster describe the appearance of a mineral, and streak describes the color of the powdered mineral.
- Each mineral has a characteristic density.
- Mohs Hardness Scale is used to compare the hardness of minerals.
- The way a mineral cleaves or fractures depends on the crystal structure of the mineral.
- Some minerals have special properties that can be used to help identify the mineral.