Introduction to Matter (Book)
Types of Matter
Elements and Atoms
The smallest particle of an element that still has the element’s properties is an atom. All the atoms of an element are alike, and they are different from the atoms of all other elements. For example, atoms of gold are the same whether they are found in a gold nugget or a gold ring (see Figure below). All gold atoms have the same structure and properties.
Gold is gold no matter where it is found because all gold atoms are alike.
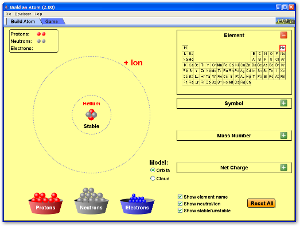
PhET Activity
Build an Atom
|
Click on image to build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas!
|